In the United States, emergency approval for a corona drug has been withdrawn for the time being – apparently because it does not work against the omicron subvariant XBB.1.5. This was announced by the US Food and Drug Administration (FDA) on Thursday.
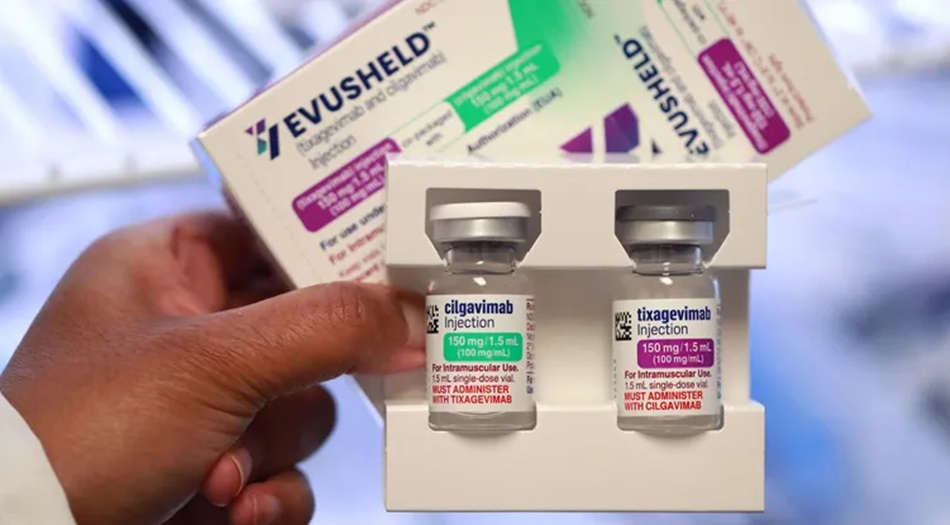
The preparation called “Evusheld” consists of the antibodies: tixagevimab and cilgavimab. The antibodies can bind to two different sites on the spike protein, i.e. the component with which the coronavirus docks to the human host cells. This is to prevent the virus from entering the host cell.
“Evusheld”: The risk of side effects should be prevented
Because of the mode of action, it is the only drug used to prevent corona. It is also approved in the EU. However, the Omicron sub-variant XBB.1.5 seems to be able to circumvent this mechanism as well, which does not ensure adequate protection.
In addition to the lack of effectiveness, the FDA also wants to prevent the risk of potential side effects by withdrawing the emergency approval, as the statement states. This can include allergic reactions, which can be serious.
Scientists suspect that the mutant has “a transmission advantage over other sublines and clear immune escape properties,” as the RKI writes in its weekly report. This also means that vaccinations may not be as effective as with previous variants. And that could become a problem.
Because XBB.1.5 – also called “Kraken” – is spreading more and more. In the current weekly report of the Robert Koch Institute (RKI) of January 26, 2023, the proportion of the variant in Germany in the week from January 9 to 15 is given as almost five percent, a significant increase compared to the previous week with a proportion of just over two Percent.
According to the American health authority CDC, in the USA, where the subvariant was first identified, it already accounts for half of all Covid infections. Current forecasts even assume that the proportion of mutants in the States could soon be 90 percent.
 Bornavirus in Bavaria: Experts sound the alarm
Bornavirus in Bavaria: Experts sound the alarm PCOS self-test: How do you know if you have PCOS?
PCOS self-test: How do you know if you have PCOS? New Corona variant XEC on the rise: more flu-like and more contagious
New Corona variant XEC on the rise: more flu-like and more contagious Bloated stomach like you’re pregnant: Causes and help
Bloated stomach like you’re pregnant: Causes and help