Turkish ray therapy gives hope for COVID-19 treatment
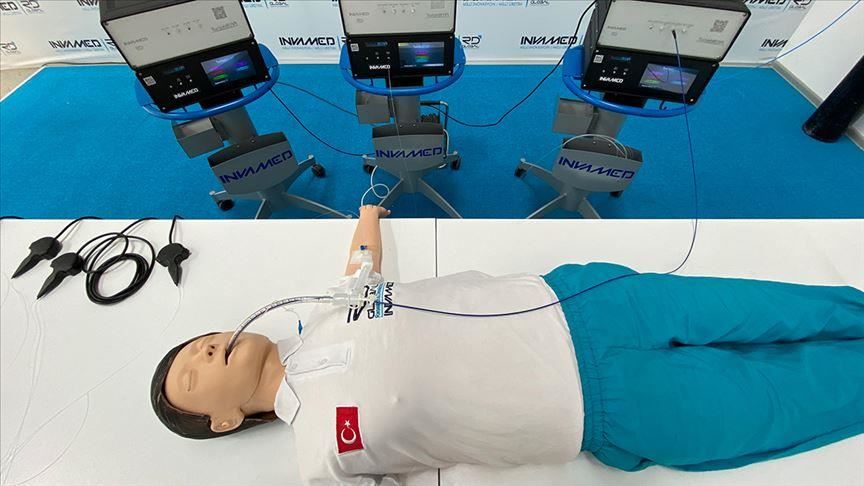
“Clinical study process, which allows implementation on patients, has started for the TurkishBeam treatment method after its application to the [Turkish] Health Ministry was approved,” cardiovascular surgeon Hikmet Selcuk Gedik at Ankara’s Gazi University said in a statement released by RD Global INVAMED.
TurkishBeam is a ray treatment system first established and developed by the world famous Turkish firm after three years of research by scientists under the name TurkishBeam Selective-Sensitive UVC and Laser Therapy, or TurkishBeam.
The method, which can be used inside the human body for the first time, is viewed as a global move by Turkey that will change paradigms.
The process started with studies on microorganisms, such as viruses and bacteria, and continued with toxicological analyses. It reached the clinical study phase after the completion of studies on animals and human cell culture.
Since November 2019, the group focused on studies on COVID-19 using the same method.
On May 4, the Health Ministry approved the application for clinical studies. After it was announced to international medical authorities, it was officially invited for testing and cooperation by the Cleveland Clinic in the US and the University of New York.
The treatment ensures the complete destruction of organisms such as microorganisms, fungi, bacteria and viruses and it does not damage cells and the DNA of humans, according to researchers.
In April, the clinical study phase officially began after its international patent application was submitted.





